Structure of the Ryanodine Receptor/Calcium Release Channel
Ryanodine receptors (RyRs) mediate the rapid release of calcium (Ca2+) from intracellular stores into the cytosol, which
is essential for numerous cellular functions including excitation–contraction coupling in muscle. Lack of sufficient
structural detail has impeded understanding of RyR gating and regulation. Here we report the closed-state structure
of the 2.3-megadalton complex of the rabbit skeletal muscle type 1 RyR (RyR1), solved by single-particle cryo-electron
microscopy at an overall resolution of 4.8A° We fitted a polyalanine-level model to all 3,757 ordered residues in each
protomer, defining the transmembrane pore in unprecedented detail and placing all cytosolic domains as tertiary folds.
The cytosolic assembly is built on an extended a-solenoid scaffold connecting key regulatory domains to the pore. The
RyR1 pore architecture places it in the six-transmembrane ion channel superfamily. A unique domain inserted between
the second and third transmembrane helices interacts intimately with paired EF-hands originating from the α-solenoid
scaffold, suggesting a mechanism for channel gating by Ca2+.
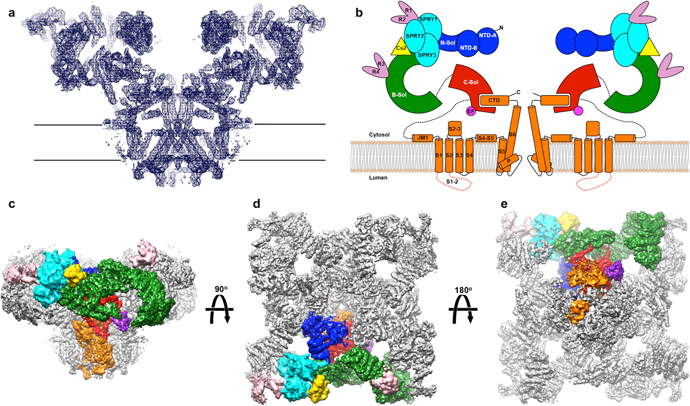
The architecture of RyR1 at 4.8A° . (a) View from the plane of
the sarcoplasmic reticulum membrane of a slab of density (blue mesh)
coinciding with the channel axis. (b) Colour-coded schematic representation of
the RyR1. B-sol, bridge solenoid; C-sol, core solenoid; N-sol, N-terminus
solenoid. (c) Viewin the plane of the sarcoplasmic reticulummembrane. (d) View
from the cytosol. (e) View from the lumen of the density map of skeletal muscle
RyR1 at 5.0° resolution, with one protomer segmented according to the
domains assigned in the model, coloured as follows: blue, N-terminal domain;
cyan, SPRY1, SPRY2 and SPRY3; salmon, clamp region (RY12 repeats), and
phosphorylation domain (RY34 repeats); yellow, calstabin; green, the bridge
solenoid scaffold; red, the core solenoid; and orange, transmembrane and
C-terminal domains; purple, putative Ca2+-binding domain (EF). Dashed lines
represent major disordered segments. LINK TO ARTICLE